The Challenge
Critical Scale Challenges
- 50+ international brands requiring consistent yet flexible design guidance
- 100,000+ global employees potentially impacted by design system decisions
- Outdated tooling (Sketch) incompatible with enterprise collaboration needs
- Security constraints around cloud-based design tools in pharmaceutical industry
- Leadership bandwidth focused on team dynamics rather than system infrastructure
The Perfect Storm: Documentation from 2018, development-focused portal alienating designers, tribal knowledge being lost as team members left, and enterprise-scale complexity across pharmaceutical brands.
Research & Discovery
Survey Research
Comprehensive assessment to 30+ teammates across disciplines using Microsoft Forms
User Interviews
1:1 moderated interviews with designers, developers, and design system users
Card Sort Analysis
Hybrid card sort to reveal user-preferred organizational models
Industry Research
Competitive analysis of documentation patterns from leading design systems
Critical Documentation Gaps Identified
Missing Component States
Component interaction states weren't documented anywhere
No Anatomical Alignment
Designers and developers used different terminology for component elements
Structural Specifications Missing
Internal and external component structure wasn't documented
Solution Strategy
Comprehensive Documentation Redesign
I redesigned the entire documentation approach to serve both audiences effectively while maintaining the system's integrity across pharmaceutical brands.
❌ Before: Legacy Portal Problems
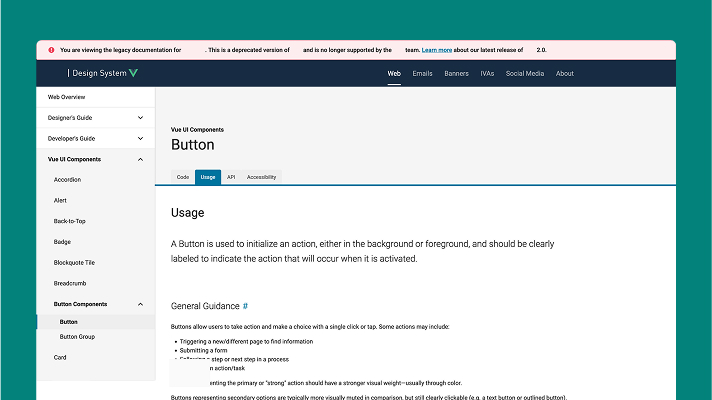
- Dense, text-heavy documentation
- Poor visual hierarchy
- Confusing navigation structure
- No component visualization
- Development-focused content
- "Got time for some light reading?"
✅ After: Redesigned Portal
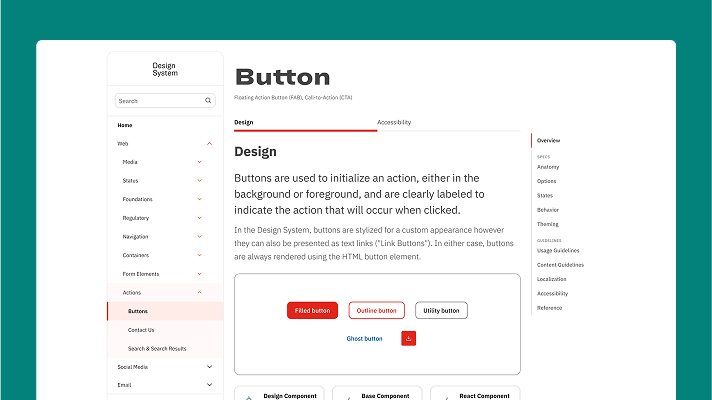
- Clean, scannable interface
- Visual component examples
- Clear anatomical breakdowns
- Multiple button states shown
- Structured sections with tabs
- Quick navigation sidebar
Specific Improvements Delivered
Content Guidelines Transformation
❌ Before
"Learn how [Brand] might be able to benefit you" - vague, confusing calls-to-action
✅ After
"Learn about [Brand]" with clear, specific action items and visual examples
Component State Documentation
❌ Before
Required manual code inspection, RGB to hex conversion, no documentation for interaction states
✅ After
Complete visual reference showing default, hover, active, focus, and disabled states for every component
Information Architecture Overhaul
❌ Before
Dense, paragraph-heavy documentation with poor scanability
✅ After
Structured content with clear section anchors, quick navigation, and visual hierarchy
Results & Business Impact
Enterprise-Scale Impact
- Multi-Brand Consistency: Streamlined design implementation across 50+ international pharmaceutical brands
- Developer-Designer Alignment: Eliminated terminology confusion through shared anatomical vocabulary
- International Design Support: Comprehensive RTL and localization guidance
- Compliance Integration: Pharmaceutical industry-specific considerations built into guidelines
Role Evolution Through Project
Research Phase
Led comprehensive user research including surveys, interviews, card sorts, and heuristic analysis to identify critical documentation gaps
Design & Product Phase
Transitioned to product owner during maternity leave, managing backlog and stakeholder alignment while continuing design work
This role evolution demonstrated adaptability and growth from researcher to strategic leader, managing both the tactical execution and the broader product strategy simultaneously.
Challenges & Solutions
Balancing Multi-Brand Requirements
Challenge: Serving dozens of pharmaceutical brands with different visual needs while maintaining system consistency
Solution: Created flexible documentation framework that showed brand variations within consistent structural patterns
Bridging Design-Development Gap
Challenge: Two audiences with different information needs and technical vocabularies
Solution: Parallel documentation paths with shared anatomical specifications that served both perspectives
Stakeholder Alignment During Role Transitions
Challenge: Maintaining project momentum while transitioning between research, design, and product owner responsibilities
Solution: Clear documentation and communication protocols that enabled smooth handoffs and continued progress
Post-Launch Strategy & Sustainability
Continuous Improvement Framework
- User Feedback Collection: Systematic gathering and analysis of feedback to identify areas for ongoing improvement
- Content Maintenance Program: Regular documentation updates to keep pace with component evolution
- Training and Adoption Support: Structured training sessions and ongoing support for the 100,000+ employee organization
- Analytics-Driven Optimization: Built-in design system analytics enabling data-driven decisions about usage patterns